Modulating neurons in an autistic background of C. elegans using optogenetics to develop a Novel Treatment for Autism
1. Acknowledgements
This research was supported by the North Carolina School of Science and Mathematics, the Glaxo Foundation, and the Burroughs Welcome Fund. I would like to thank Dr. Kim Monahan for guiding me and for being an encouraging mentor throughout this research process. I would like to thank the Caenorhabditis Genetics Center (CGC) for providing me with the model organisms for my research. I would also like to thank Trisha Avula, Heidi Kauffman, and Dr. Scheck for providing me with extra support. Finally, I would like to thank Keven Bahena Velazquez, Ozioma Obi, Anjalee Hardy, Makena Neale, Clara Smith, and the rest of the Research in Biology class of 2023 for being extremely supportive throughout this project.
2. Abstract
Autism Spectrum disorder is a prevalent neurodevelopmental disorder characterized by social and communication deficits. Current treatments include behavioral and cognitive therapies that act to optimize the abilities of autistic individuals and mitigate the symptoms. Optogenetics, however, can be used as a viable treatment for autism as it is non-invasive and targets the etiology of the disorder through neuron modulation. Optogenetics is a method often used in neuroscience research as it allows for the manipulation of specific neurons, proteins, and genes. An aldicarb assay will be performed on Caenorhabditis elegans (C. elegans) to study behavioral changes and neuron function induced by optogenetic treatment. C. elegans are commonly used in neuroscience research because of their similarities to the human nervous system. The genes being studied in the experiment include nmr-2 and egl-19; egl-19 and nmr-2 are homologs to human genes associated with autism. A paralysis assay was performed to evaluate how neurotransmission differs between the autism-associated genes with or without blue light treatment. This will allow changes in the rate of synaptic transmission to be analyzed, which can allude to the optogenetic treatment’s effectiveness. This research is critical because it is a novel method to treat autism. I was the primary and sole researcher for this experiment. I wrote my own proposal, paper, developed my procedure, and conducted the experiment under the guidance of my research mentor. I also presented this research at a variety of conferences.
3. Introduction
3.1. Autism
Autism Spectrum Disorder is a highly prevalent neurodevelopmental disorder that affects 1 in every 44 children. The term “Autism Spectrum Disorder” is used to describe a broad range of conditions characterized by cognitive impairments such as repetitive behaviors, hindered social skills, and communication deficits. “People with ASD also have an increased risk of psychiatric problems such as anxiety, depression, obsessive-compulsive disorder, and eating disorders” [3]. Because of its prevalence, we are now able to identify Autism symptoms as young as 18 months old.
Researchers are unaware of a “single cause” for Autism and have speculated that genetics might be a primary factor as certain genes have appeared to be associated with autism. They have also suggested that certain genetic mutations might increase the risk of a child having Autism and are continuing to explore whether environmental factors such as pollution or infection are associated with Autism [4].
Common “treatments” for Autism include different forms of therapy such as cognitive, occupational, and behavioral therapy as well as certain medications. These treatments, however, act to mitigate the symptoms and work to help autistic individuals optimize their abilities, rather than the etiology of the disorder [4].
3.2. C. elegans
Caenorhabditis elegans, or C. elegans, are non-parasitic nematodes commonly used in neuroscience research. C. elegans have a small, well-studied nervous system, comprised of only 302 neurons. Several other characteristics including transparency, genetic conservation, and similarities to the human nervous system make them ideal model organisms for neuroscience research. C. elegans have also been previously used in optogenetic studies of the nervous system. Despite the complexity of their nervous system, their processes have been thoroughly studied to reveal information about neuron and gene expression [42].
3.3. Autism-associated Genes
Studies have shown that certain genes and mutations are more prevalent in individuals with Autism [33]. Nmr-2, a homolog of the human gene GRIN2B, functions as an NMDA receptor, which is also associated with glutamate production. Glutamate is an excitatory neurotransmitter for which deficits have been found to pertain to individuals with Autism.
Nmr-2 is a synaptic mutant and has been found to have “a role in coordinating
progeny-induced social behavior” [31]. In addition, egl-19, the C. elegans homolog to the human gene CACNA1C, provides instructions for making calcium voltage-gated channels. Calcium channels help with the cell’s ability to create electrical impulses and are crucial for the function of nerve cells. Additionally, they aid in the regulation of certain genes that aid in brain development. Mutations of CACNA1C are associated with autism, but further research is needed to confirm these correlations.
3.4. Optogenetics
Optogenetics is a technique used to control the function of neurons, proteins, and genes using light and opsins. Opsins can be excitatory or inhibitory ion channels or ion pumps that open or close through the absorption of different wavelengths of light. QW309 is the strain that contains neurons that have been genetically modified to contain Channelrhodopsin-2, a light-sensitive algal protein derived from Chlamydomonas reinhardtii. ChR2, “a cation channel that opens in the presence of blue light (∼470 nm) to depolarize neurons,” is the most used excitatory opsin [13]. “ChR2 is expressed mainly in C. reinhardtii under low-light conditions, suggesting involvement in photoreception in dark-adapted cells” [13]. The neurons of organisms that possess ChR2 can be perturbed (inhibited) or stimulated through the use of blue light.
Utilizing optogenetics, I will study and modify the function of the neurons where these genes are expressed. Many previous experiments involving optogenetics have been performed using mice and other neurodegenerative diseases such as Parkinson’s and Alzheimer’s. For example, one study used optogenetics to test if transplanted dopamine neurons could be controlled to restore motor function in Parkinson’s mice. They found that transplantation of dopamine neurons did improve motor function. The results also suggested that optogenetics could be used to develop therapy where dopaminergic neurons could be inhibited to prevent dyskinesia, a common Parkinson’s motor symptom, or neurons could be stimulated to provide therapeutic relief. More research is required to determine the effectiveness of this approach, however, optogenetics has proved to be a viable technique [9]. Moreover, optogenetics is a very novel technique being used that is still being improved to help further the neuroscience field [1].
3.5. Hypothesis
Alternate Hypothesis: C.elegans that have knocked down nmr-2 or egl-19, will elicit behavioral changes when exposed to blue light as demonstrated by the aldicarb-paralysis assay.
Null Hypothesis: Blue light will not induce a change in the function of neurons and therefore not cause behavioral changes in C.elegans presenting with Autism Spectrum Disorder.
4. Methods
4.1. C. elegans strains and maintenance
C. elegans were grown and maintained on 50mm Nematode Growth Media (NGM) plates seeded with 0.05 mL of E.coli OP50 at room temperature (15 ºC). Different strains from the Caenorhabditis Genetics Center were studied: N2 (wild-type) and QW309 (zfIs18).
4.2. Feeding RNAi
Overnight cultures of bacteria expressing different dsRNA were cultured and seeded on RNAi plates. L4 QW309 worms were placed onto the RNAi food to induce acute transient knockdown of the following genes: egl-19, nmr-2, tom-1, and pos-1.
Worms fed pos-1 (control) dsRNA bacteria would experience embryonic arrest, which would ensure that fRNAi was effective.
4.3. ATR and Bluelight Activation
Multiple experimental trials were performed to analyze the effects of blue light and to eliminate external variables. For optogenetic trials, plates were prepared with or without all-trans-retinal (ATR). ATR is a co-factor required for blue light activation of ChR2. When ATR was included, 2µl of ATR stock solution (100mM ATR in ethanol) was added to 2000µl of either respective fRNAi or OP50. Plates were then seeded with 50µl of this solution and stored under aluminum foil to prevent external light from altering data.
4.4. Aldicarb-Induced Paralysis Assay
Aldicarb is an acetylcholinesterase inhibitor that can be used to analyze synaptic transmission in C. elegans. “In the presence of aldicarb, acetylcholine continues to accumulate, causing persistent muscle contraction and eventual paralysis”[2]. NGM agar plates containing 1mM aldicarb were prepared and stored at 4°C until use the next day. NGM aldicarb plates were then seeded with 20µl OP50 E.coli and cultured at room temperature (20 °C) overnight. 5-10 L4 worms were transferred onto aldicarb-containing plates and tested for paralysis every 30 minutes until every worm had been scored as paralyzed. If they had not become paralyzed within 500 minutes, the worms were recorded as being resistant to aldicarb. To test for paralysis, worms were prodded with the tip of a platinum wire pick.
Worms fed tom-1 (control) should show a paralysis time, as knockdown of tom-1 causes C. elegans to be more sensitive to aldicarb.
4.5. Statistical Analysis
A survival analysis was performed to plot survival graphs to illustrate the rates of paralysis for each group of worms using GraphPad Prism. Overall survival was used as the endpoint in the analysis and was not statistically significant.
5. Results
5.1 Data
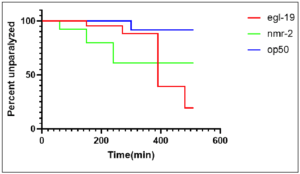
Figure 1: Knockdown of autism genes egl–19 and nmr–2 caused decreased time to
paralysis in worms, indicating that there was a significant (p–value of 0.0335)
decrease in synaptic transmission. QW309 worms fed bacteria seeded with OP50
served as the control group. Worms fed bacteria seeded with egl–19 and nmr–2 served
as experimental groups. Decrease in synaptic transmission corresponds with the
literature for C.elegans with the Autism Spectrum Disorder phenotype. Figure displays
percent of C. elegans that were unparalyzed at every 20 minute time point over the course of 600 minutes. Analysis and figure created using GraphPad Prism.
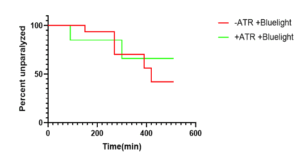
Figure 2: P-value was not significant indicating that ATR(all-trans-retinal) did not act as an external factor and therefore didn’t influence the data acquired when blue light was administered. (+)ATR group was fed bacteria seeded with ATR and OP50. ATR is an important cofactor used along with blue light for proper optogenetic stimulation. Figure displays percent of QW309 C. elegans that were unparalyzed at every 20 minute time point over the course of 600 minutes. Analysis and figure created using GraphPad Prism.
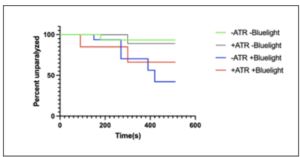
Figure 3: OP50 data was significant with p-value of 0.0324 indicating that blue light has a significant effect on synaptic transmission in QW309 worms fed OP50. Worms fed OP50 served as the control group, as opposed to worms fed knockdown gene. ATR(+) worms were fed bacteria seeded with ATR and OP50. ATR is an important cofactor used along with blue light for proper optogenetic stimulation. Worms who were stimulated with blue light and fed ATR had a significant difference in paralysis time when compared with other groups. Figure displays percent of C. elegans that were unparalyzed at every 20-minute time point over the course of 600 minutes. Analysis and figure created using GraphPad Prism.
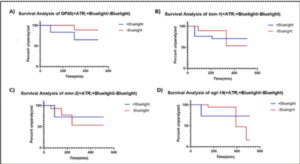
Figure 4: This figure depicts the results of the aldicarb assay performed on each strain after blue light was administered vs no blue light. QW309 worms were fed bacteria seeded with all-trans-retinal (ATR). OP50 worms served as the controls and were fed bacteria seeded with OP50. Nmr-2 and egl-19 worms were fed RNAi bacteria. ATR is an important cofactor used along with blue light for proper optogenetic stimulation. Figure displays percent of C. elegans that were unparalyzed at every 20-minute time point over the course of 600 minutes. Analysis and figure created using GraphPad Prism.
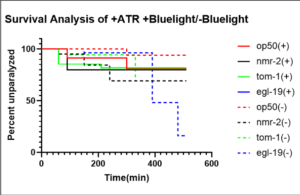
Figure 5: This survival analysis graph depicts the difference in rate of paralysis caused by blue light in worms fed bacteria seeded with all-trans-retinal (ATR). The solid lines indicate that blue light was administered (+), while the dotted lines indicate that blue light was not administered (-). OP50 worms served as the controls and were fed bacteria seeded with OP50. Nmr-2 and egl-19 worms were fed RNAi bacteria. ATR is an important cofactor used along with blue light for proper optogenetic stimulation. Figure displays percent of C. elegans that were unparalyzed at every 20-minute time point over the course of 600 minutes. Analysis and figure created using GraphPad Prism.
To study the effects of blue light on synaptic transmission, an aldicarb-induced paralysis assay was performed. The survival analyses for OP50 worms were compared to those of egl-19 and nmr-2 when treated +/- blue light and +/- ATR. Increased time to paralysis indicates that synaptic transmission was increased. Conversely, decreased paralysis time indicates that synaptic transmission was decreased. Each graph depicts the probability of C. elegans survival in comparison to time in minutes. Over 50 percent of OP50, nmr-2, and tom-1 worms were left unparalyzed by the end of the 500 minutes.
6. Discussion
6.1. Conclusion
Figure 1 compares the results of the aldicarb-induced paralysis assay that was performed for each of the 3 QW309 strains mutated through fRNAi but not treated with blue light. The survival analysis yielded a significant p-value (0.035) indicating that there was a decrease in synaptic transmission in comparison to worms that did not have knocked down autism-associated genes. Further trials were conducted to ensure that ATR and OP50 were not responsible for any changes that could be attributed to blue light. Similarly, Figure 2 depicts the results of the aldicarb assay performed on OP50 worms that were all treated with bluelight but varied in regard to ATR. The data yielded a non-significant p-value indicating that ATR did not act as an external confounding variable. Figure 3 depicts the results of the aldicarb assay and compares how worms fed OP50 responded after being exposed to +bluelight or -blue light in relation to the converse treatment. The results of this experiment were significant with a p-value of 0.0324 indicating that bluelight influences synaptic transmission in QW309 worms fed OP50. Figure 4 portrays the survival proportions of all the strains treated with blue light and fed ATR in comparison with those not treated with blue light but still fed ATR. Figure 5 contains the survival analysis curves for all the strains of QW309 worms fed ATR. The results presented in Figure 5 yielded a nonsignificant p-value suggesting that if a correlation lies between blue light treatment and synaptic transmission, it is very small. The p-values demonstrate that blue light was not an effective treatment for Autism in this experiment.
6.2. Limitations
Due to this research being conducted at a high school lab, there were many limitations including funding, available equipment, and restrictions for model organisms to be used. For many optogenetic experiments, researchers utilized specific lasers that allowed for more precise activation of specific neurons. These lasers are extremely expensive and can be difficult to acquire in a high school setting. Additionally, many optogenetic experiments are often conducted using mice as a model organism. However, only invertebrates were allowed for this experiment, which posed difficulties as more optogenetic literature and procedures have used mice as opposed to C. elegans as a model organism. Also, C. elegans with Channelrhodopsin-2(ChR-2) in autism-associated neurons have not been developed. Instead, it was assumed neurons activated by blue light would affect the function of neurons implicated in Autism. If this research were to be reconducted in the future, it would be beneficial to acquire a professional optogenetic laser. It would also be beneficial to use C. elegans strains containing ChR-2 in autism-associated neurons to allow for acute activation of specific neurons, which will help mitigate any external variability and improve the accuracy of the results produced.
6.3. Future Directions
With this knowledge, developing a model organism that possesses ChR2 in autism-associated neurons would be necessary to establish optogenetics as a viable treatment for Autism. Additionally, further assays would need to be performed to ensure that synaptic transmission was altered by blue light treatment.
